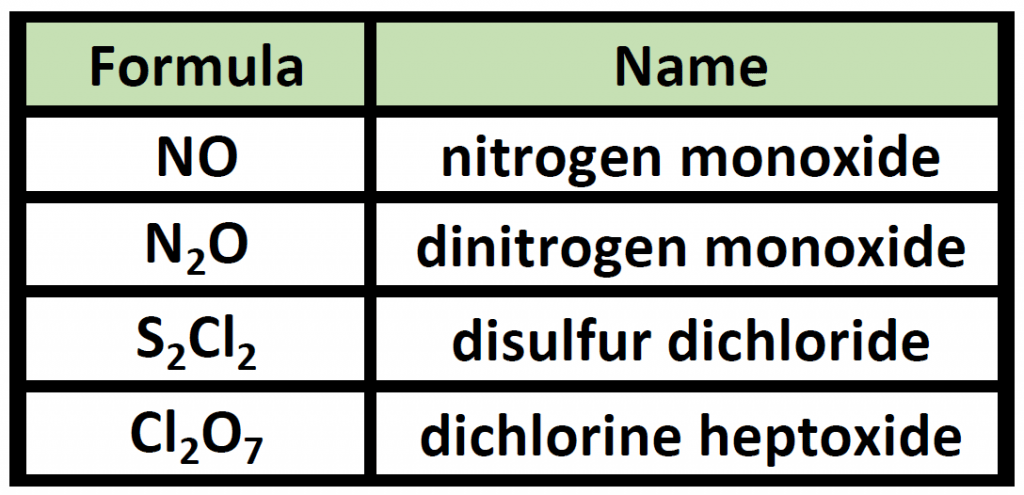
How Are Covalent Bonds Named
Naming covalent compounds Covalent bonds nonpolar molecule How does the strength of a covalent bond relate to its length?
Covalent Compounds - Examples and Properties
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry Covalent examples compounds compound properties Reading: covalent bonds
Question #4fc53 + example
Covalent ionic compounds kovalen molecule ikatan chemistry nonpolar coordinate bonding senyawa h2o molecules atom chemische bindung kimia nitrogen hcl confusedCovalent compounds chemistry examples molecular names table naming formulas bonds molecules prefix compound binary ionic general nitrogen two ch150 elements Covalent naming compounds rules nomenclatureBond strength covalent length single bonds vs double triple two relate does its longest socratic weakest size atoms involves electrons.
Covalent compoundsCovalent ionic bonding bonds electrons formation formed atoms differences chemistry stable Covalent vs ionic bond- definition, 11 key differences, examples.